Pipeline
TheraVectys develops lentiviral vectors to prevent and treat cancer and infectious diseases,
both for human and veterinary indications.
TheraVectys’ mission is to provide solutions for oncological and infectious disease indications through therapeutic, prophylactic as well as personalized vaccination. TheraVectys supports its development through international academic and industrial collaborations with European and Chinese partners.
In June 2017, TheraVectys and Pasteur Institute created a Joint-Laboratory to focus the company on its core business, pre-clinical research and reinforce collaboration with the Institut Pasteur, ensuring the continuous follow-up of current proof of concept studies while building a solid portfolio of potential candidates for clinical developments.
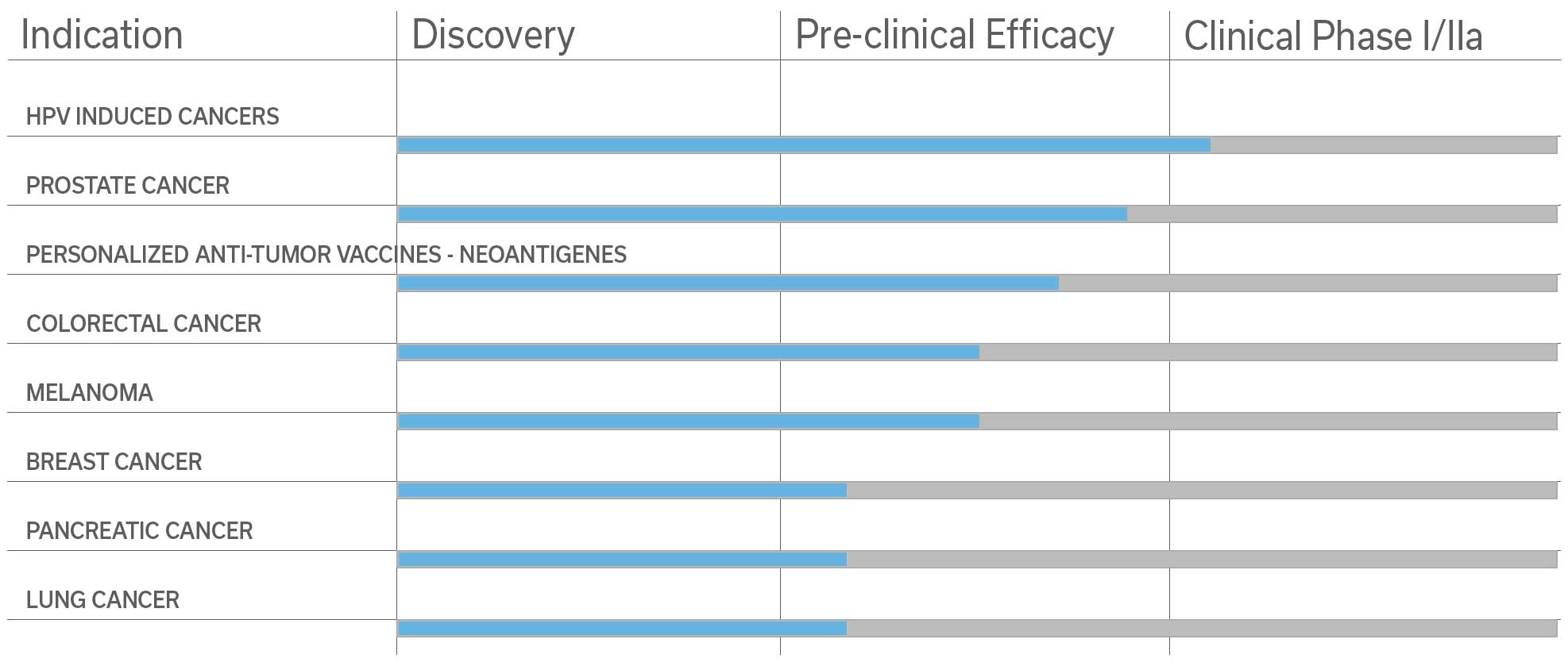
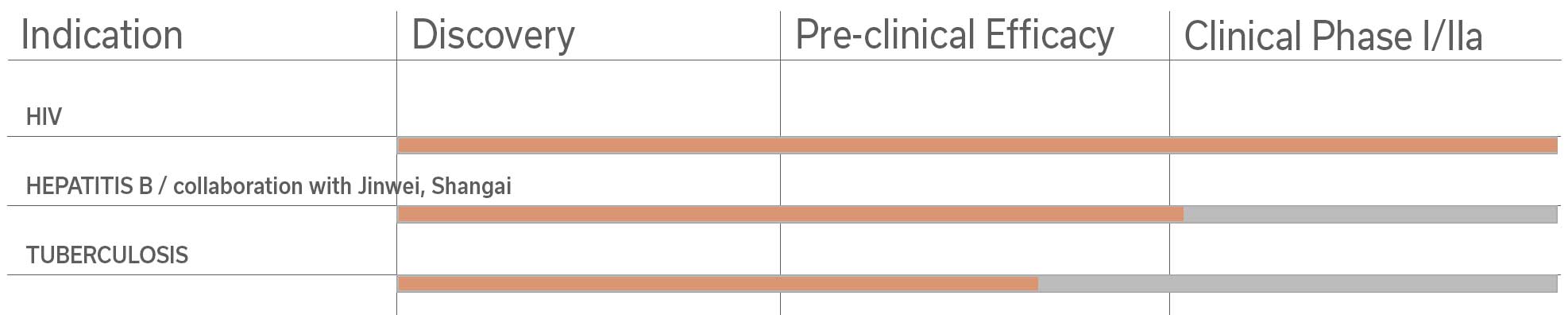
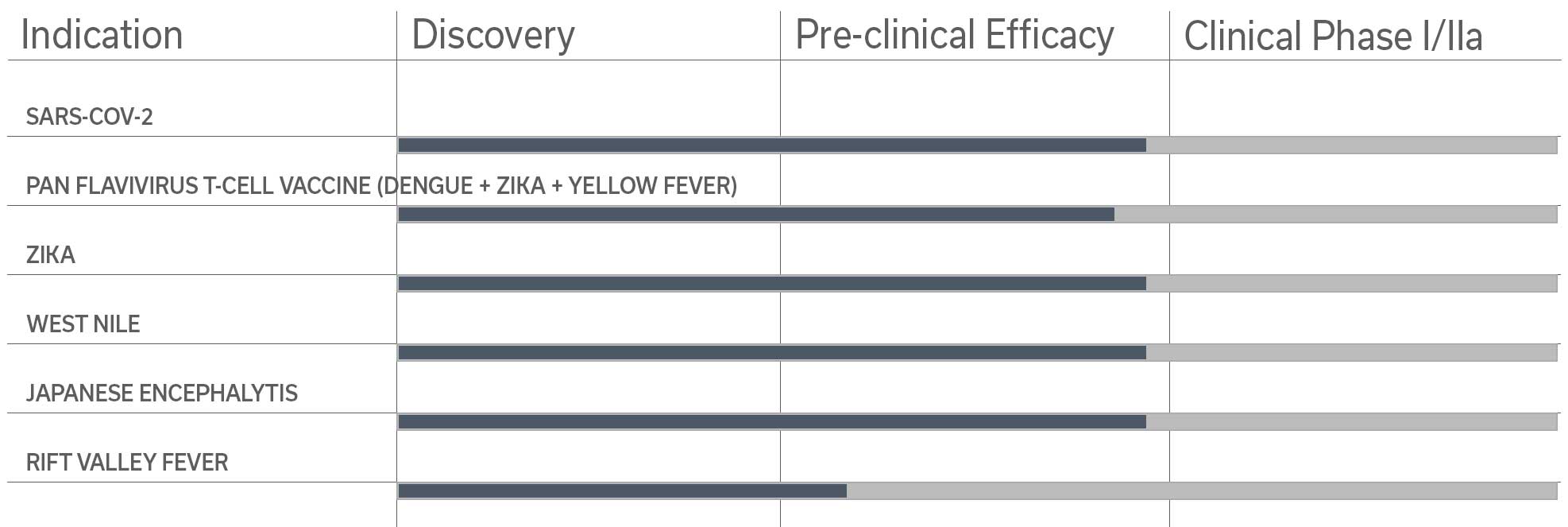
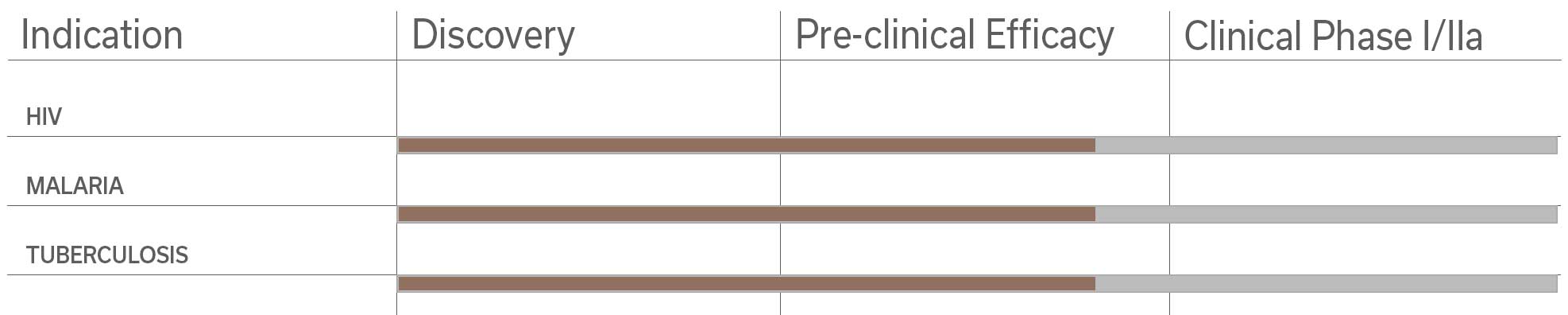
Among the pre-clinical studies and proofs of concept studies conducted by the Pasteur Institute-TheraVectys Joint-Laboratory, TheraVectys has selected 8 major indications for pre-clinical and clinical developments for the coming years.
It illustrates the potential of its vaccine platform in oncology and infectious diseases, which is based both upon the very promising results obtained by existing proof of concept animal studies, and the market interests materialized by collaboration agreements signed with major partners in 2017 and 2018.
TheraVectys strong ambition to be recognized as a leading therapeutic oncological platform is supported by recent proof of concept results. In the foreseeable future, TheraVectys intends to directly or via collaboration prepare or launch 5 clinical trials.
TheraVectys envisages to start pre-clinical processes for Phase I clinical trials on:
- Sars-Cov-2
- HPV-induced cancers (cervix and oropharyngeal cancer)
- Prostate cancer
In parallel, phase I clinical trials on hepatitis B and liver cancer will be conducted in China by TheraVectys partner Shanghai Jinwei Biotechnology under two existing collaboration agreements. Following analysis presents estimated projections for all of them based on European or US sources.