Technology
TheraVectys develops pioneering T cell vaccines which targets dendritic cells in-vivo.
Our goal is to profoundly transform global health.
TheraVectys’ technology has an almost unlimited number of applications in infectious diseases and cancers, and is probably the biggest silent revolution in the vaccination field.
Lentiviral Vector Immunotherapy
The ultimate paradigm shift: A breakthrough technology to cure cancers and prevent infectious diseases.
UNPRECEDENTED RESULTS FOR PREVIOUSLY UNTREATABLE DISEASES
This groundbreaking approach led to unparalleled results, such as:
- Complete protection against SIV (the HIV equivalent in monkeys)
- Complete neutralization of SARs-CoV2 antigen through nasal instillation
Efficacy and safety
Through pre-clinical data generated over 12 years: TheraVectys has demonstrated the efficacy and safety of lentiviral vector-based vaccines in a wide variety of diseases and with different animal models. These pre-clinical results point to promising results in humans and encourage us to begin clinical trials.
Proprietary technology
We have a worldwide license from the Institut Pasteur to exclusively develop, manufacture and use lentiviral vector-based vaccines.
VIRAL VECTORS:
FROM VIRUS TO VACCINE
Viruses enter the cells of host organisms and deliver genetic material. Viral vectors exploit this ability.
Viral vectors are derived from viruses that have been engineered to lose their pathogenic potential as well as being able to transport and deliver the payload (genetic material) into the host cells. This can be used to repair a deficient gene (gene therapy) or to induce an immune response (vaccination).
Vaccine candidates based on viral vector can stimulate two types of adaptive immune responses:
Humoral response
Production of specific antibodies that neutralize or damage pathogens
Cell-mediated response
Stimulation of specific T cells that destroy cancer cells or those infected with a pathogen
DENDRITIC CELLS: A KEY
TO CELL-MEDIATED IMMUNITY
Dendritic cells trigger immune responses by processing antigens derived from pathogens and turning them into segments, which they present at their surface to specific T cells inside the local lymph nodes or other secondary lymphoid organs.
This antigen presentation induces a specific cell-mediated response via the clonal proliferation of T cells, able to highly specifically recognize the infected or cancer cells.
As a result, dendritic cells have become a major target for new vaccines intended to induce a cell-mediated immune response.
HOW LENTIVIRAL VECTOR TECHNOLOGY WORKS
Lentiviral vectors stimulate dendritic cells to efficiently train T cells, which can recognize antigens and eliminate antigen-bearing cells, such as infected or cancerous cells.
Effective and long-lasting immune prevention or immunotherapies need to trigger not only antibody responses but also a strong T-cell response, including cytotoxic T cells (a cell-mediated response) to destroy cancer cells or cells that are infected with pathogens.
WHAT MAKES OUR LENTIVIRAL VECTORS SO REMARKABLE?
- They are non integrative and non replicative (enhanced safety)
- Have a strong tropism for dendritic cells
- Can mount a strong, diversified, efficient and long-term immune response
- They are very weakly inflammatory, which paves the way for their use in mucosal vaccination, for instance by nasal instillation
THE DNA FLAP: PROPELLING LENTIVIRAL VECTORS FORWARD
Lentiviral vectors are presently the only viral vectors able to efficiently deliver
their genetic material within the nucleus of the dendritic cells.
Lentiviral Vector Proprietary Platform
Fully optimized lentiviral vector immunotherapy platform
The most efficient
and safest platform by design
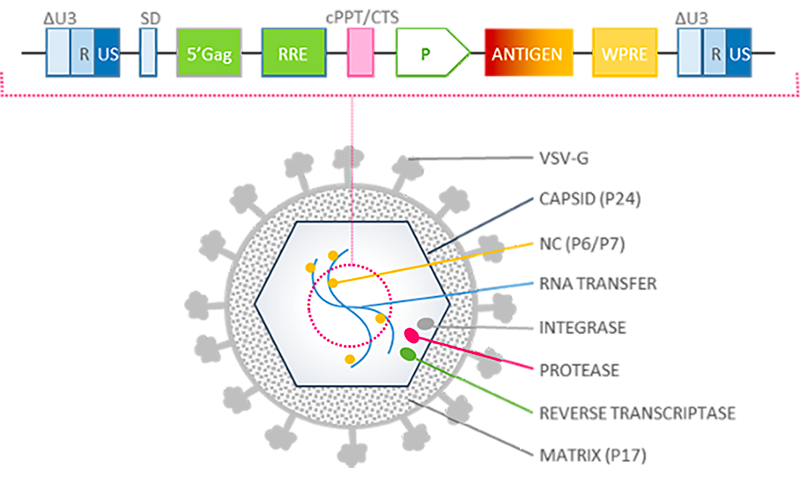
Our lentiviral vector platform attributes:
- Non-replicative
- Non-integrative
- Non-pathogenic
- Optimized internal promoter
- Wide expression cassette enabling complex, multi-epitopic immunogen design
- Enhanced tropism for dendritic cells through VSV pseudotype envelopes
- No pre-existing immunity against vector VSV pseudotypes
- Sustained endogenous presentation of antigens
- Prime/boost and single injection regimens
- No adjuvant
- Low production costs due to low active dose
- Easy distribution through dry freezing process
- Very weakly inflammatory
Safety demonstrated through quantitative biodistribution studies
and one phase I/IIa study
Our first-in-human phase I/IIA was performed with no serious adverse event.
ClinicalTrials.gov Identifier: NCT02054286
Spectacular efficacy studies
An intense, broad, and long-lasting T-cell immune response
This includes endogenous antigen processing leading to sustained presentation through the whole dendritic cell life. The physiological but weak maturation of dendritic cell means that there is no need for adjuvant. Long-term T-cell immunity is challenged in various animal models including through lethal challenges.
Challenged against most deadly untreated pathogens
Our focus was to challenge against deadly untreated pathogens, this led us to some unparallel results.We had the best protection ever described in a predictive macaque/SIVmac 251 model, with 100% protection against SIV, the macaque equivalent of HIV. In terms of Malaria, we had a unique long-term sterile cellular immunity by a liver-stage vaccine. Our work with Zika produced long-term sterilizing protection based on a single injection. This vaccine demonstrated superiority to plasmid and RNA vaccines.
(Reference)
These groundbreaking in vivo preclinical results favorably position our vaccine candidates for encouraging results in human clinical assays.
The lentiviral approach demonstrates results in cancer studies
Our approach for treating cancers has produced impressive results in animal models with complete tumor eradication based on a single vaccination.
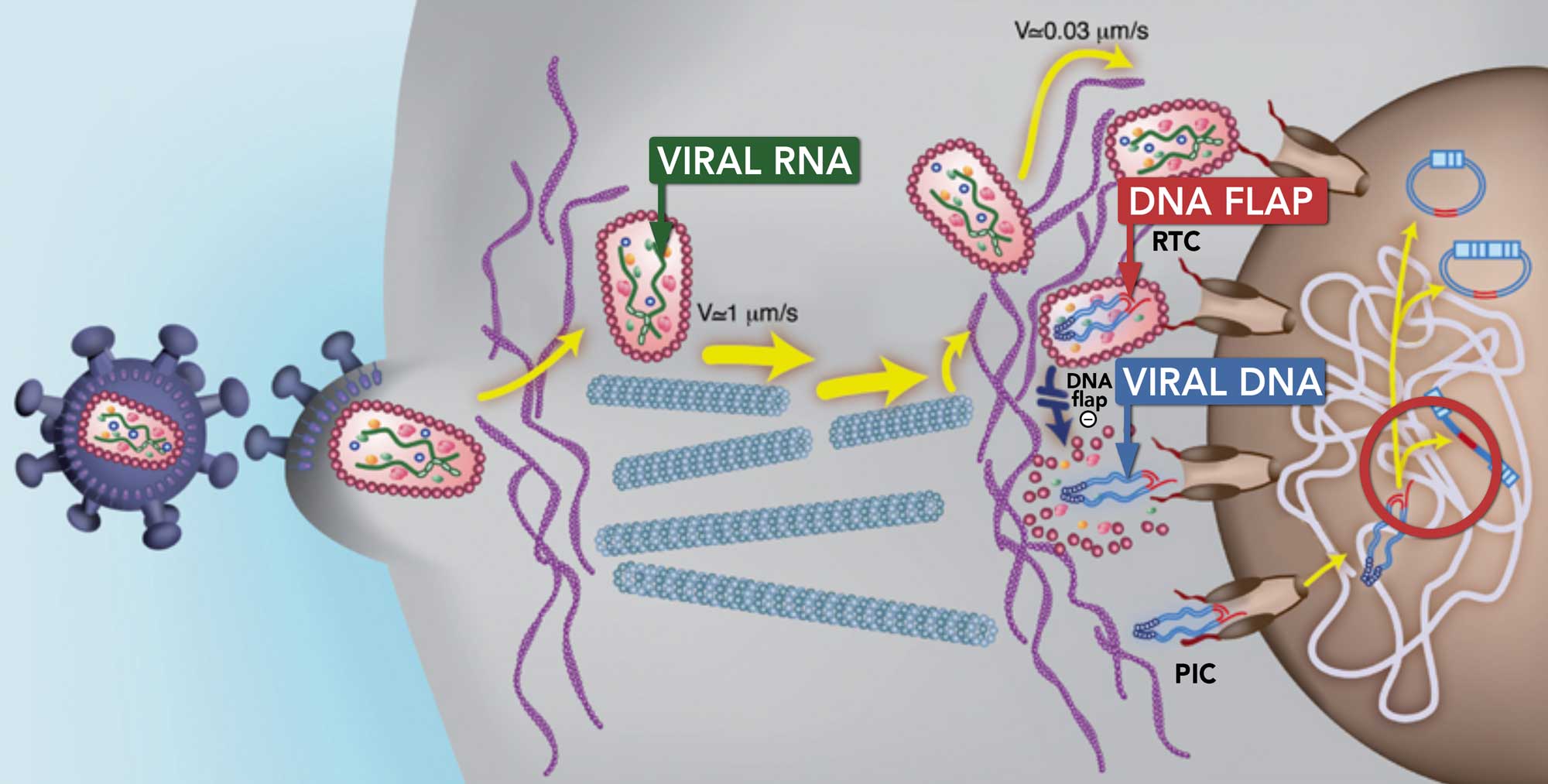
The DNA Flap
The DNA flap propelled the lentiviral vector field forward
Lentiviral vectors are the only viral vectors able to efficiently deliver
their genetic material within the nucleus of the dendritic cells.
Investigations conducted by Dr. Pierre Charneau (TheraVectys’s founder) at the Molecular Virology and Vaccinology research unit
of the Institut Pasteur led to the discovery of the DNA flap (Triple-stranded DNA with a length of 99 nucleotides).
The DNA flap is part of the viral DNA which allows it to cross the host cell nuclear membrane to enter the nucleus.
In this way, lentiviruses can also transduce non-dividing cells, including dendritic cells.
TheraVectys has developed a new generation of prophylactic and therapeutic vaccines
and immunotherapies based on the use of lentiviral vectors.
TheraVectys’ proprietary lentiviral vector technology is continuously optimized to deliver unrivalled capacity to induce strong,
efficient and long lasting cellular immune responses and humoral response.
A pioneering discovery
What is the DNA flap?
- The DNA flap is a triple DNA helix structure formed out of viral DNA before the virus enters the nucleus.
- The DNA flap is the final product of HIV reverse transcription.
- More specifically, when the viral RNA is converted into viral DNA during reverse transcription, a linear DNA molecule with a central plus-strand overlap is created, forming the DNA flap.

This image illustrates the DNA flap process (Image: EMBO Journal)
The DNA flap enables the lentiviral DNA to cross the nuclear membrane and enter the cell nucleus. In this way, lentiviruses can also transduce non-dividing cells whereas other retroviruses cannot transduce non-dividing cells.